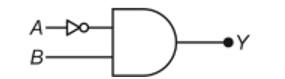
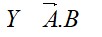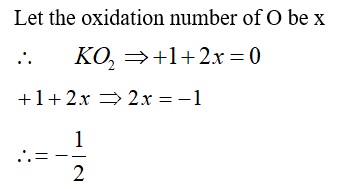11.30. A certain salt X, gives the following results.
(i) Its aqueous solution is alkaline to litmus.
(ii) It swells up to a glassy material Y on strong heating.
(iii) When conc.H2SO4is added to a hot solution of X, white crystal of an acid Z separates out.
Write equations for all the above reactions and identify X, Y and Z. (Advance)
11.30. A certain salt X, gives the following results.
(i) Its aqueous solution is alkaline to litmus.
(ii) It swells up to a glassy material Y on strong heating.
(iii) When conc.H2SO4is added to a hot solution of X, white crystal of an acid Z separates out.
Write equations for all the above reactions and identify X, Y and Z. (Advance)
The compounds X, Y and Z are borax, sodium metaborate + boric anhydride and boric acid respectively.
When borax is heated, it first swells and then forms a transparent glass like bead of sodium meta borate and boric anhydride.
Na2B4O7 à2NaBO2+B2O3+10H2O
(Borax) (sodium metaborate) (Boric anhydrid
Similar Questions for you
From BF3 to BI3 Lewis acidic strength increases
F2 is the strongest oxidising agent
HClO4 is the most acidic compound.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering