11.45 When 3-methylbutan-2-ol is treated with HBr, the following reaction takes place: Give a mechanism for this reaction. (Hint : The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.

Give a mechanism for this reaction. (Hint : The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.
11.45 When 3-methylbutan-2-ol is treated with HBr, the following reaction takes place: Give a mechanism for this reaction. (Hint : The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.

Give a mechanism for this reaction. (Hint : The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.
-
1 Answer
-
11.45The first step in the mechanism of the given reaction is protonation of the alcohol followed by loss of water to give a 20 carbocation.
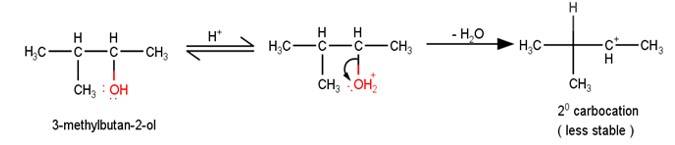 A
AThe next step is a rearrangement of the 20 carbocations formed in the above step is less stable it rearranges by a 1,2-hydride shift to form more stable 3° carbocations.
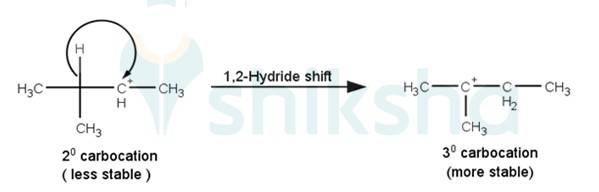
The last step of the reaction is the nucleophilic attack of Br- ion on the 3° carbocations giving the final product.
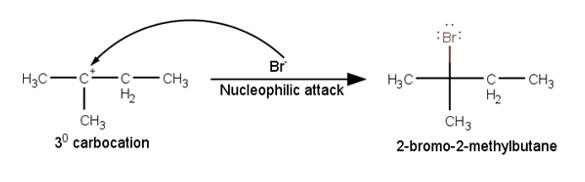
Similar Questions for you
Rainbow is formed due to internal reflection and dispersion.
Correct order of acidic strength
Correct order of acidic strength
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers







