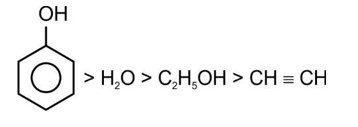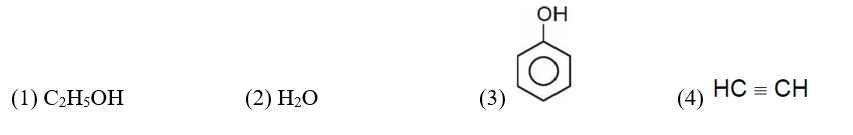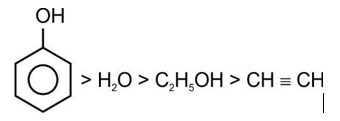11.70 Give equations of the following reactions: (i) Oxidation of propan-1-ol with alkaline KMnO4 solution. (ii) Bromine in CS2 with phenol. (iii) Dilute HNO3 with phenol. (iv) Treating phenol with chloroform in presence of aqueous NaOH.
11.70 Give equations of the following reactions: (i) Oxidation of propan-1-ol with alkaline KMnO4 solution. (ii) Bromine in CS2 with phenol. (iii) Dilute HNO3 with phenol. (iv) Treating phenol with chloroform in presence of aqueous NaOH.
-
1 Answer
-
11.70
Oxidation of propane-1-ol with alkaline KMnO4 solution gives propanoic acid as the product. As the oxidation of primary alcohol gives carboxylic acid as the major product in the presence of a strong oxidizing reagent. And here KMnO4 is a very strong oxidizing agent.
A mixture of o-bromo phenol and p-bromo phenol is formed.
The formation of 2 products depends totally on the reaction conditions.
Dilute HNO3 with phenol.
Only dilute acid will be required for the nitration of phenol, nitric acid contains a small amount of nitrous acid which because of the activation of the ring will be more than enough to nitrate the phenol. Two products
...more
Similar Questions for you
Rainbow is formed due to internal reflection and dispersion.
Correct order of acidic strength
Correct order of acidic strength
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers