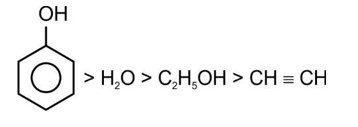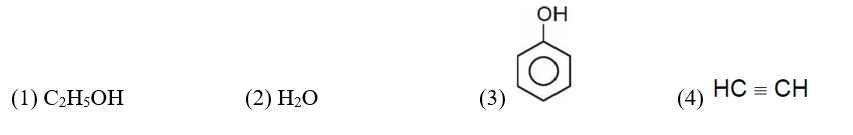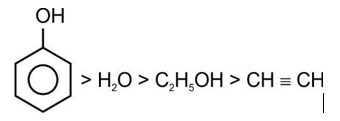11.82 Explain the fact that in aryl alkyl ethers (i) The alkoxy group activates the benzene ring towards electrophilic substitution and (ii) It directs the incoming substituents to ortho and para positions in benzene ring.
11.82 Explain the fact that in aryl alkyl ethers (i) The alkoxy group activates the benzene ring towards electrophilic substitution and (ii) It directs the incoming substituents to ortho and para positions in benzene ring.
-
1 Answer
-
11.82
(i) In aryl alkyl ethers the +R effect of the alkoxy group leads to an increase in the electron density of the benzene ring as they push electrons into the ring making the benzene ring activated towards electrophilic substitution reactions. This could be understood more clearly from the following resonating structures : -
(ii) It could be clearly seen from the above resonating structures that the electron density increases more at the ortho and para positions as compared to the meta positions. Hence, we can conclude that the alkoxy group directs the incoming substituents to ortho and para positions in the benzene ring.
For example
...more
Similar Questions for you
Rainbow is formed due to internal reflection and dispersion.
Correct order of acidic strength
Correct order of acidic strength
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers