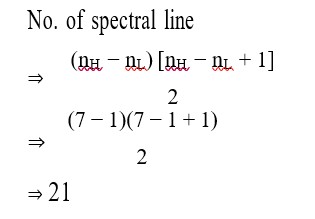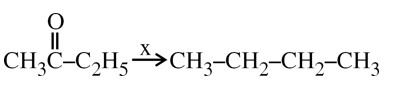12.12. What are electrophiles and nucleophiles? Explain with examples.
12.12. What are electrophiles and nucleophiles? Explain with examples.
-
1 Answer
-
Electrophiles: The name electrophiles mean electron loving. Electrophiles are electron deficient. They may be positive ions or neutral molecules.
Ex: H+, Cl+, Br+, NO2+, R3C+, RN2+, AlCl3, BF3
Nucleophiles: The name nucleophiles means 'nucleus loving' and indicates that it attacks the region of low electron density (positive centres) in a substrate molecule. They are electron rich they may be negative ions or neutral molecules.
Ex: Cl– Br–, CN–, OH–, RCR2–, NH3, RNH2, H2O, ROH etc.
Similar Questions for you
Kindly consider the solution
Fact.
No. of spectral line
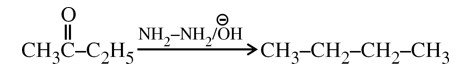
Wolf Kishner Reduction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers