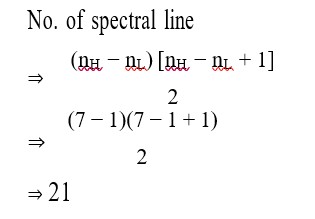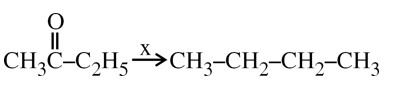12.14. Classify the following reactions in one of the reaction type studied in this unit.
(a) CH3CH2Br + HS– → CH3CH2SH + Br–
(b) (CH3)2C=CH2 + HCl → (CH3)2ClC—CH3
(c) CH3CH2Br + HO– → CH2=CH2 + H2O + Br–
(d) (CH3)3C—CH2OH + HBr → (CH3)2CBrCH2CH2CH3 + H2O
12.14. Classify the following reactions in one of the reaction type studied in this unit.
(a) CH3CH2Br + HS– → CH3CH2SH + Br–
(b) (CH3)2C=CH2 + HCl → (CH3)2ClC—CH3
(c) CH3CH2Br + HO– → CH2=CH2 + H2O + Br–
(d) (CH3)3C—CH2OH + HBr → (CH3)2CBrCH2CH2CH3 + H2O
-
1 Answer
-
(a) Nucleophilic substitution (b) Electrophilic addition
(c) Bimolecular elimination (d) Nucleophilic substitution with rearrangement.
Similar Questions for you
Kindly consider the solution
Fact.
No. of spectral line
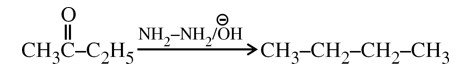
Wolf Kishner Reduction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers