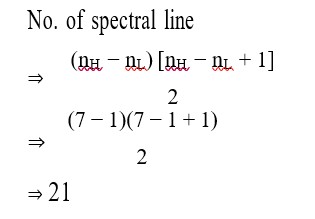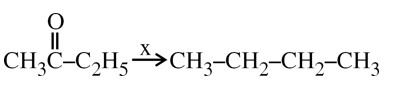12.17. Explain the terms Inductive and Electromeric effects. Which electron displacement effect explains the following correct orders of acidity of the carboxylic acids?
(a) Cl3CCOOH > Cl2CHCOOH > ClCH2 COOH
(b) CH3CH2COOH > (CH3)2 CHCOOH > (CH3)3CCOOH
12.17. Explain the terms Inductive and Electromeric effects. Which electron displacement effect explains the following correct orders of acidity of the carboxylic acids?
(a) Cl3CCOOH > Cl2CHCOOH > ClCH2 COOH
(b) CH3CH2COOH > (CH3)2 CHCOOH > (CH3)3CCOOH
-
1 Answer
-
Inductive effect: The inductive effect refers to the polarity produced in a molecule as a result of higher electronegativity of one atom compared to another. Atoms or groups which lose electron towards a carbon atom are said to have +I Effect. Examples of +I effect are (Electron releasing)
(CH3)2C—, (CH3)2CH—, CH3CH2— CH3— etc.
Those atoms or groups which draw electron away from a carbon atom are said to have -I Effect. Examples of -I effect are:
NO2, F, Cl, Br, I, OH etc.
Electromeric effect: The electromeric effect refers to the polarity produced in a multiple bonded compound as it is approached by a reagent.
The atom A has lost...more
Similar Questions for you
Kindly consider the solution
Fact.
No. of spectral line
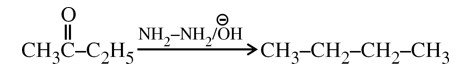
Wolf Kishner Reduction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers