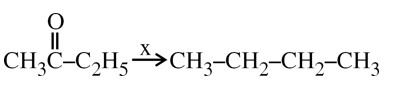12.21. Discuss the chemistry of Lassaigne’s test.
12.21. Discuss the chemistry of Lassaigne’s test.
-
1 Answer
-
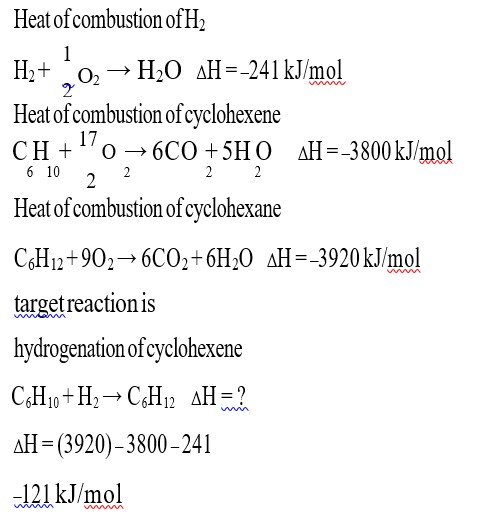
Lassaigne's test: In organic compounds, nitrogen, sulphur and halogens are covalently bonded. Their detection in 'Lassaigne's test' is possible if they are in the ionic form. This can be achieved by fusing the organic compound with sodium metal.
Chemistry for test for nitrogen:
Sodium fusion extract is boiled with ferrous sulphate and acidified with sulphuric acid. Sodium cyanide reacts with ferrous sulphate and forms sodium hexacyanoferrate (II). On heating with sulphuric acid, some ferrous is oxidized to ferric hexacyanoferate (II) Fe4 [Fe (CN)6]3 which is prussian blue in colour.
Chemistry of the test for sulphur:
Aceti
...more
Similar Questions for you
Kindly consider the solution
Fact.
No. of spectral line
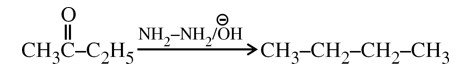
Wolf Kishner Reduction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers