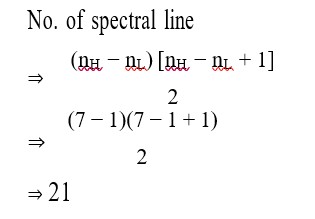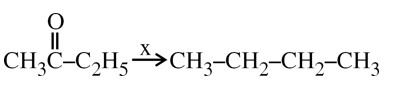12.22. Differentiate between the principle of estimation of nitrogen in an organic compound by (i) Dumas method (ii) Kjeldahl’s method.
12.22. Differentiate between the principle of estimation of nitrogen in an organic compound by (i) Dumas method (ii) Kjeldahl’s method.
-
1 Answer
-
(i) Dumas method: The nitrogen containing organic compound, when heated with copper oxide in an atmosphere of carbon dioxide, yields free nitrogen in addition to carbon dioxide and water
(ii)Kjeldahl's method: A known mass of the organic compound is heated strongly with conc. H2SO4, a little potassium sulphate and a little mercury (a catalyst). As a result, the nitrogen present in the organic compound is converted to ammonium sulphate.
Similar Questions for you
Kindly consider the solution
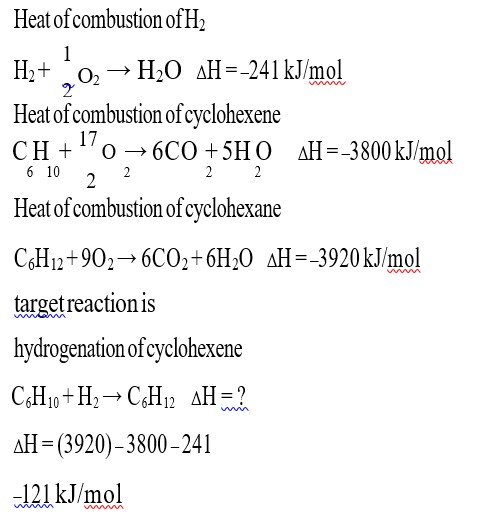
Fact.
No. of spectral line
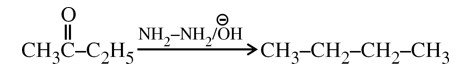
Wolf Kishner Reduction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers