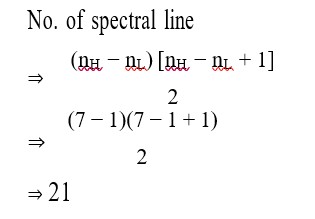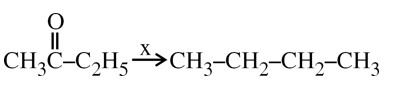12.32. An organic compound contains 69% carbon and 4.8% hydrogen, the remainder being oxygen. Calculate the masses of carbon dioxide and water produced when 0.20 g of this compound is subjected to complete combustion.
12.32. An organic compound contains 69% carbon and 4.8% hydrogen, the remainder being oxygen. Calculate the masses of carbon dioxide and water produced when 0.20 g of this compound is subjected to complete combustion.
-
1 Answer
-
Step 1: Calculation of mass of CO2 produced
Mass of compound = 0.20 g
% of carbon = 69%
i.e. 12/44 x = Mass of Carbondioxide formed / Mass of Compound = 69/100
Therefore, mass of CO2formed = (69 x 44 x 0.20) / (12 x 100) = 0.506 g
Step 2: Calculation of mass of H2O produced
Mass of compound = 0.20 g
% of hydrogen = 4.8%
i.e. 2/18 x Mass of Water Formed/Mass of Compound = 4.8/100
Therefore, mass of H2O formed = 4.8*18*0.20/2*100 = 0.0864 g
Similar Questions for you
Kindly consider the solution
Fact.
No. of spectral line
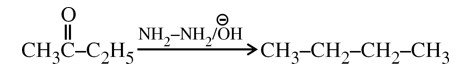
Wolf Kishner Reduction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers