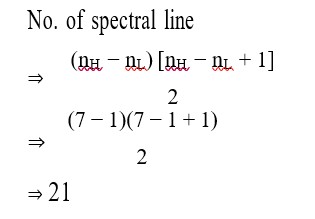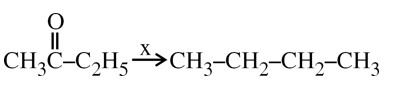12.33. A sample of 0.50 g of an organic compound was treated according to Kjeldahl’s method. The ammonia evolved was absorbed in 50 ml of 0.5M H2SO4. The residual acid required 60 ml of 0.5 M solution of NaOH for neutralization. Find the percentage composition of nitrogen in the compound.
12.33. A sample of 0.50 g of an organic compound was treated according to Kjeldahl’s method. The ammonia evolved was absorbed in 50 ml of 0.5M H2SO4. The residual acid required 60 ml of 0.5 M solution of NaOH for neutralization. Find the percentage composition of nitrogen in the compound.
-
1 Answer
-
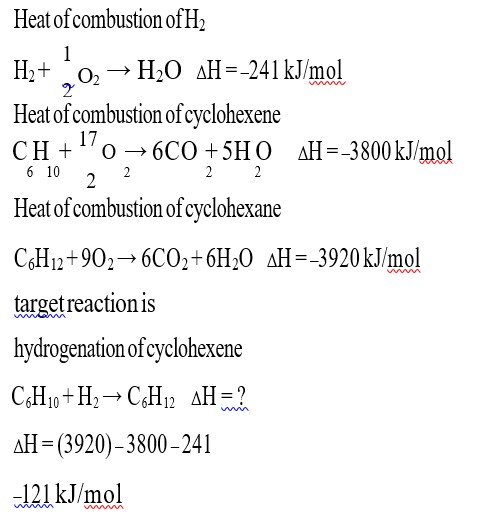
Step I: Calculation of volume of unused acid i.e. V2 =?
V1 = Volume of NAOH solution required = 60 cm3
N1 = Normality of NaOH solution = ½ N
N2 = Normality of H2SO4 = 1N
Applying N1V1 = N2V2
½ N x 60 cm3 = 1N x V2
Or V2 = 30 cm3
Step II: calculation of volume of acid used
Volume of acid added = 50 cm3
Volume of unused acid = 30 cm3
Volume of acid used = 50 – 30 = 20 cm3
Step III: Calculation of % of nitrogen
Mass of compound = 0.50 g
Volume of acid used = 20 cm3
Normality of acid used = 1 N
% of nitrogen = (1.4 x 20 x 1) / 0.50 = 56%
Similar Questions for you
Kindly consider the solution
Fact.
No. of spectral line
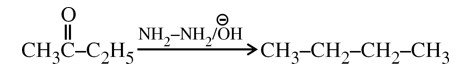
Wolf Kishner Reduction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers