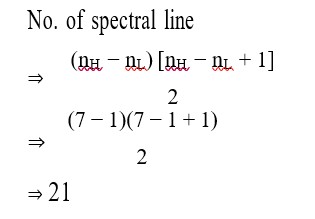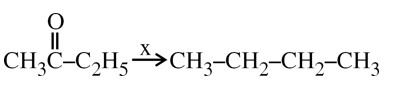12.44. Assertion: The energy of actual structure of the molecule(the resonance hybrid) is lower than that of anyof the canonical structures.
Reason: Resonance is particularly important when the contributing structures are equivalent in energy.
12.44. Assertion: The energy of actual structure of the molecule(the resonance hybrid) is lower than that of anyof the canonical structures.
Reason: Resonance is particularly important when the contributing structures are equivalent in energy.
-
1 Answer
-
(b) The difference in energy between the actual structure and the lowest energy resonance structure is called the resonance stabilisation energy or simply the resonance energy. The more the number of important contributing structures, the more is the resonance energy.
Similar Questions for you
Kindly consider the solution
Fact.
No. of spectral line
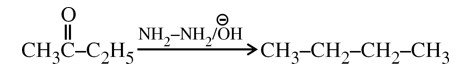
Wolf Kishner Reduction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers