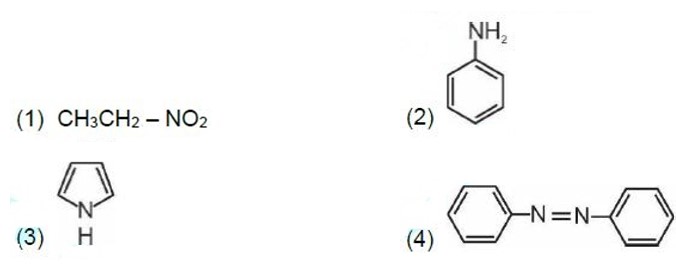
13.13 Arrange the following:
(i) In decreasing order of the pKb values: C2H5NH2 , C6H5NHCH3 , (C2H5 ) 2NH and C6H5NH2
(ii) In increasing order of basic strength: C6H5NH2 , C6H5N(CH3)2 , (C2H5)2NH and CH3NH2
(iii) In increasing order of basic strength: (a) Aniline, p-nitroaniline and p-toluidine (b) C6H5NH2, C6H5NHCH3 , C6H5CH2NH2
(iv) In decreasing order of basic strength in gas phase: C2H5NH2 , (C2H5)2NH, (C2H5)3N and NH3
(v) In increasing order of boiling point: C2H5OH, (CH3 )2NH, C2H5NH2
(vi) In increasing order of solubility in water: C6H5NH2 , (C2H5 ) 2NH, C2H5NH2 .
13.13 Arrange the following:
(i) In decreasing order of the pKb values: C2H5NH2 , C6H5NHCH3 , (C2H5 ) 2NH and C6H5NH2
(ii) In increasing order of basic strength: C6H5NH2 , C6H5N(CH3)2 , (C2H5)2NH and CH3NH2
(iii) In increasing order of basic strength: (a) Aniline, p-nitroaniline and p-toluidine (b) C6H5NH2, C6H5NHCH3 , C6H5CH2NH2
(iv) In decreasing order of basic strength in gas phase: C2H5NH2 , (C2H5)2NH, (C2H5)3N and NH3
(v) In increasing order of boiling point: C2H5OH, (CH3 )2NH, C2H5NH2
(vi) In increasing order of solubility in water: C6H5NH2 , (C2H5 ) 2NH, C2H5NH2 .
Pkb value is the negative logarithm of the basicity constant (Kb) .i.e., pKb = -logKb
Evidently, smaller the value of pKb , stronger is the base (strong tendency to donate electrons). Aliphatic amines(R-NH2) are more basic(tendency to donate electrons) than aromatic amines(C6H5NH2) because of the fol
Similar Questions for you
In Amines, the nitrogen atom bonds with alkyl or aryl groups replacing hydrogen, whereas in amides, the nitrogen atom bonds directly with the carbonyl group (-CO-).
Kjeldahl's method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in the ring.
Correct order of basic strength in aqueous medium is
Kindly consider the following figure
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering