13.22 Write the reactions of
(i) Aromatic
(ii) Aliphatic primary amines with nitrous acid.
13.22 Write the reactions of
(i) Aromatic
(ii) Aliphatic primary amines with nitrous acid.
(i) Aromatic amines react with nitrous acid (prepared in situ fromNaNO2 and a mineral acid such as HCl) at 273 - 278 K to form stable aromatic diazonium salts i.e., NaCl and water. This reaction is widely used for preparation of variety of compounds.
(ii) Aliphatic primary amines react with nitrou
Similar Questions for you
In Amines, the nitrogen atom bonds with alkyl or aryl groups replacing hydrogen, whereas in amides, the nitrogen atom bonds directly with the carbonyl group (-CO-).
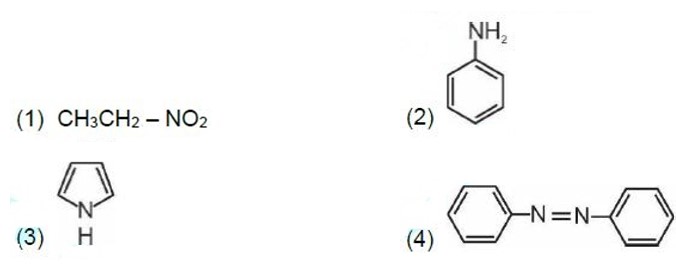
Kjeldahl's method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in the ring.
Correct order of basic strength in aqueous medium is
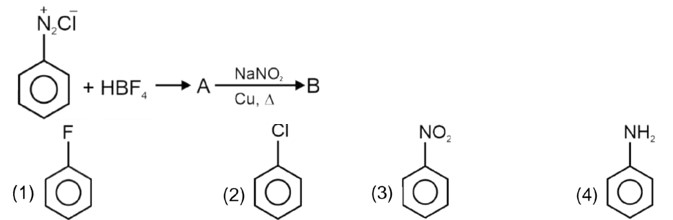
Kindly consider the following figure
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering