14.1 Glucose or sucrose is soluble in water but cyclohexane or benzene (simple six membered ring compounds) is insoluble in water. Explain.
14.1 Glucose or sucrose is soluble in water but cyclohexane or benzene (simple six membered ring compounds) is insoluble in water. Explain.
14.1
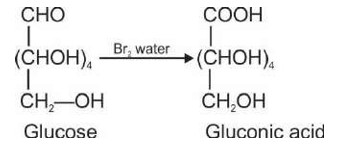
Glucose and sucrose are carbohydrates (optically active polyhydroxy aldehydes or ketones).
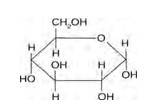
Structure of glucose:
Structure of sucrose:
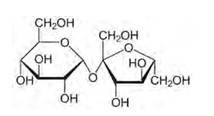
As you can see both the compounds have five –OH and eight –OH groups respectively. These –OH groups are responsible for the extensive hydrogen bonding with water.
Similar Questions for you
Insulin is a globular proteins.
Ionisation enthalpy increases in a period. Z dominates over screening effect (s) in a period as Zeff. increases.
Kindly go through the solution
Histidine is an essential amino acid
Lactose is a disaccharide which is formed by forming C? -C? glycosidic linkage between D-galactose and D- glucose.
Lactose - (Hydrolysis)-> D - galactose + D - glucose
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering