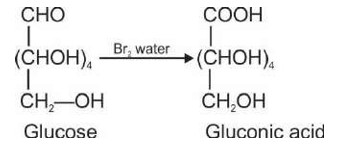
14.3 How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
14.3 How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
14.3
D-glucose reacts with hydroxylamine (NH2OH) to form oxime due to the presence of the aldehyde functional group (-CHO). This is due to the cyclic structure of glucose which forms an open chain structure in an aqueous medium, which then reacts to give an oxime.
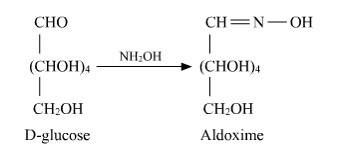
But in case of pentaacetate of D-glu
Similar Questions for you
Insulin is a globular proteins.
Ionisation enthalpy increases in a period. Z dominates over screening effect (s) in a period as Zeff. increases.
Kindly go through the solution
Histidine is an essential amino acid
Lactose is a disaccharide which is formed by forming C? -C? glycosidic linkage between D-galactose and D- glucose.
Lactose - (Hydrolysis)-> D - galactose + D - glucose
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering