3. At temperatures above 1073K coke can be used to reduce FeO to Fe. How can you justify this reduction with the Ellingham diagram?
3. At temperatures above 1073K coke can be used to reduce FeO to Fe. How can you justify this reduction with the Ellingham diagram?
-
1 Answer
-
3. From Ellingham diagram, we know that ΔG° (C, CO) < ΔG° (Fe, Fe), the following reactions are:
C+ O2→CO
2Fe+O2→2FeO
Therefore, FeO can be reduced to Fe by coke.
Similar Questions for you
is the temperature Co-efficient of cell. The cell having less variation of EMF, with respect to temperature have high efficiency.
Below 1350° C, Mg can reduce Al2O3 and above 1350°C, Al can reduce MgO (from Ellingham diagram).
Melting and boiling point of Mg are lower than that of Al.
In ores/mineral available earthy and undesired impurities are gangue
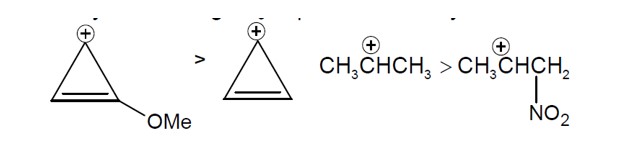
Sol. Reactivity towards depend on stability of carbocation formed
Leaching involves the given reaction,
Here, O2 is required for formation of Au (l) cyanide complex but no complex in absence of O2.
In above displacement reaction, Zn is oxidized.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers