4.1. Explain the formation of a chemical bond.
4.1. Explain the formation of a chemical bond.
-
1 Answer
-
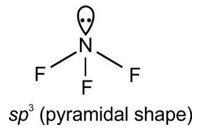
4.1. Chemical bond is an attractive force which binds atoms, ions etc. together in a compound. According to Kossel and Lewis, atoms combine together in order to complete their respective octets so as to acquire the configuration of the nearest stable inert gas. This can occur in two ways; by transfer of one or more electrons from one atom to other or by sharing of electrons between two or more atoms. The chemical bond formed by sharing of electrons is called a covalent bond. In this process a chemical bond is formed.
Similar Questions for you
He2 has zero bond order hence it does not exist.
The three fundamental laws of chemistry are - Law of Definite Proportions, Law of Conservation of Mass, and Law of Multiple Proportions.
The three types of chemical bonds are - ionic, metallic and covalent bonds. When the electrons transfer between the atoms, they form the Ionic bonds by producing charged ions that are attracted to each other. When atoms share electrons, covalent bonds are created. When metal atoms share a sea of delocalized electrons, metallic bonds get created.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers