4.16. Write the significance/applications of dipole moment.
4.16. Write the significance/applications of dipole moment.
-
1 Answer
-
4.16. Significance of dipole moment are:
In predicting the nature of the molecules: Molecules with specific dipole moments are polar in nature and those of zero dipole moments are non-polar in nature.
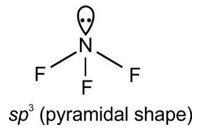
In the determination of shapes of molecules.
In calculating the percentage ionic character.
Similar Questions for you
He2 has zero bond order hence it does not exist.
The three fundamental laws of chemistry are - Law of Definite Proportions, Law of Conservation of Mass, and Law of Multiple Proportions.
The three types of chemical bonds are - ionic, metallic and covalent bonds. When the electrons transfer between the atoms, they form the Ionic bonds by producing charged ions that are attracted to each other. When atoms share electrons, covalent bonds are created. When metal atoms share a sea of delocalized electrons, metallic bonds get created.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers