4. Wrought iron is the purest form of iron. Write a reaction used for the preparation of wrought iron from cast iron. How can the impurities of sulfur, silicon and phosphorus be removed from cast iron?
4. Wrought iron is the purest form of iron. Write a reaction used for the preparation of wrought iron from cast iron. How can the impurities of sulfur, silicon and phosphorus be removed from cast iron?
-
1 Answer
-
4. Iron oxides are mixed with limestone and coke in order to decompose carbonates and oxidize sulfides. This mixture is then fed into the blast furnace. Many reactions take place in different temperature ranges of the blast furnace. By oxidizing impurities from cast iron in a reverberatory furnace which is lined by hematite, wrought iron or malleable iron is obtained. This is the purest form of commercial iron. This can be shown with the following reaction:
Fe2O3 + 3C → 2Fe + 3CO.
Sulfur, silicone and phosphorus are oxidized and passed into the slag and removed as impurities when limestone is added as a flux to it.
Similar Questions for you
is the temperature Co-efficient of cell. The cell having less variation of EMF, with respect to temperature have high efficiency.
Below 1350° C, Mg can reduce Al2O3 and above 1350°C, Al can reduce MgO (from Ellingham diagram).
Melting and boiling point of Mg are lower than that of Al.
In ores/mineral available earthy and undesired impurities are gangue
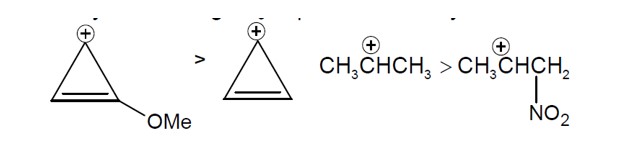
Sol. Reactivity towards depend on stability of carbocation formed
Leaching involves the given reaction,
Here, O2 is required for formation of Au (l) cyanide complex but no complex in absence of O2.
In above displacement reaction, Zn is oxidized.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers