43. In which of the following methods of purification, metal is converted to its volatile compound which is decomposed to give pure metal?
(i) Heating with a stream of carbon monoxide.
(ii) Heating with iodine.
(iii) Liquation.
(iv) Distillation.
43. In which of the following methods of purification, metal is converted to its volatile compound which is decomposed to give pure metal?
(i) Heating with a stream of carbon monoxide.
(ii) Heating with iodine.
(iii) Liquation.
(iv) Distillation.
-
1 Answer
-
43. Option (i) and (ii)
In the vapor phase refining method, the metal is converted into its volatile compound and then is collected elsewhere. This involves two techniques:
1. Mond Process for refining Nickel: in this process, a volatile complex, nickel tetracarbonyl is formed when nickel is heated with a stream of carbon monoxide.
2. Van Arkel Method for refining Zirconium or Titanium: this method is basically used for removal of oxygen and nitrogen present as impurities in the Zr or Ti metal. These are heated in an evacuated metal with iodine. As iodine is more covalent than these metals, it volatilizes.
Similar Questions for you
is the temperature Co-efficient of cell. The cell having less variation of EMF, with respect to temperature have high efficiency.
Below 1350° C, Mg can reduce Al2O3 and above 1350°C, Al can reduce MgO (from Ellingham diagram).
Melting and boiling point of Mg are lower than that of Al.
In ores/mineral available earthy and undesired impurities are gangue
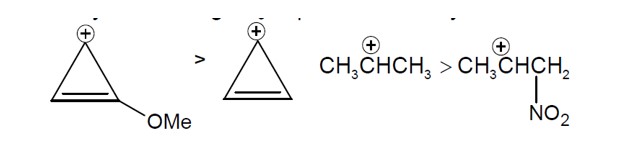
Sol. Reactivity towards depend on stability of carbocation formed
Leaching involves the given reaction,
Here, O2 is required for formation of Au (l) cyanide complex but no complex in absence of O2.
In above displacement reaction, Zn is oxidized.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers