52. Which of the following is not true about the voids formed in 3-dimensional hexagonal close packed structure?
(i) A tetrahedral void is formed when a sphere of the second layer is present above triangular void in the first layer.
(ii) All the triangular voids are not covered by the spheres of the second layer.
(iii) Tetrahedral voids are formed when the triangular voids in the second layer lie above the triangular voids in the first layer and the triangular shapes of these voids do not overlap.
(iv) Octahedral voids are formed when the triangular voids in the second layer exactly overlap with similar voids in the first layer.
52. Which of the following is not true about the voids formed in 3-dimensional hexagonal close packed structure?
(i) A tetrahedral void is formed when a sphere of the second layer is present above triangular void in the first layer.
(ii) All the triangular voids are not covered by the spheres of the second layer.
(iii) Tetrahedral voids are formed when the triangular voids in the second layer lie above the triangular voids in the first layer and the triangular shapes of these voids do not overlap.
(iv) Octahedral voids are formed when the triangular voids in the second layer exactly overlap with similar voids in the first layer.
-
1 Answer
-
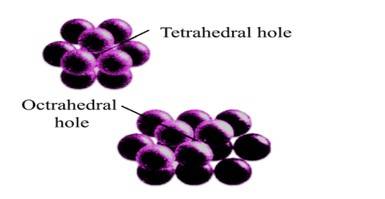
52. Option (iii) and (iv) are correct since tetrahedral voids are formed when the triangular void in the second layer lie exactly above the triangular voids in the first layer and the triangular shape of these voids oppositely overlap while the octahedral voids are formed when triangular void of second layer is not exactly overlap with similar void in first layer. This can be shown as-
Similar Questions for you
· Density of solid decreases in Schottky defect and vacancy defect.
· Density of solid increases in interstitial defect.
· Density of solid remain unchanged in Frenkel defect.
77. Option (iii) Assertion is correct statement, but reason is wrong statement is the answer since intermediate conductivity in semiconductor is due to the small energy gap between valence band and conduction band and hence the assertion is correct, but the reason is wrong.
76. Option (ii) Assertion and reason both are correct statements, but reason is not correct explanation for assertion is the answer since in fcc structure the number of atoms present = 4 per unit cell which provides a maximum packing efficiency of 74%.
75. Option (iii) Assertion is the correct statement, but the reason is wrong is the answer since besides the body centre there is one octahedral void at the centre of each 12 edges which is shared between four adjacent unit cells. Thus, Octahedral voids present at the body centre of the cube = 1
12 octahedral voids are located at each edge and shared between four-unit cells = 12 * = 3
Total number of octahedral voids = 4.
74. Option (ii) Assertion and reason both are correct statements, but reason is not correct explanation for assertion is the answer since in graphite carbon atoms are arranged in different layers and each atom is covalently bonded to three of its neighbouring atoms in the same layer due to which the fourth valence electron of each atom, present between the different layers is free to move and this makes graphite a good conductor of electricity.
As different layers can slide over each other which makes it soft. while in case of diamond C atom is bonded covalently with their adjacent atoms throughout the crystal. Thus, all the four v
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
