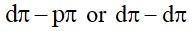6.12 Write chemical reactions taking place in the extraction of zinc from zinc blend.
6.12 Write chemical reactions taking place in the extraction of zinc from zinc blend.
-
1 Answer
-
The steps involved in the extraction of zinc from zinc blende (ZnS) are as followed:-
- Concentration: the ore is crushed and then concentrated by froth floatation
- Roasting: the concentrated ore is heated in the presence of an excess of air at about 1200K to form zinc
- ZnS + 3 O2 → 2 ZnO + 2 SO2
- Reduction: ZnO obtained above is mixed with powdered coke and heated to 1673K in a fireclay
- ZnO + C → Zn + CO
- Electrolytic Refining: Zinc is refined by the process of electrolytic refining. In this process, impure zinc is made the anode and a pure copper strip is made the cathode. The electrode used in an acidified solution of zinc sulphate (Z
...more
Similar Questions for you
Na+ C + N + S ®NaSCN
Fe3+ + SCN–
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AIF6 or CaF2 which lowers the melting point of the mixture and brings conductivity.
Ellingham diagram explains the feasibility of reduction process not the kinetics of process.
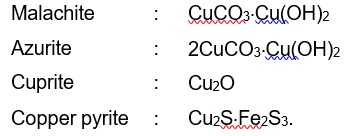
Malachite : CuCO3.Cu (OH)2
Azurite : 2CuCO3.Cu (OH)2
Cuprite : Cu2O
Copper pyrite : Cu2S.Fe2S3.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers