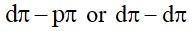6.25 The value of ?fG0 for formation of Cr2 O3 is – 540 kJmol^−1and that of Al2 O3 is – 827 kJmol^−1 . Is the reduction of Cr2 O3 possible with Al ?
6.25 The value of ?fG0 for formation of Cr2 O3 is – 540 kJmol^−1and that of Al2 O3 is – 827 kJmol^−1 . Is the reduction of Cr2 O3 possible with Al ?
-
1 Answer
-
The value of Δ fG0 for the formation of Cr2O3 is – 540 kJ mol-1 which are higher than that of Al2O3 is – 827 Kjmol-1. Thus, Al can reduce Cr2O3 to Cr. Hence, the reduction of Cr2O3 with Al is possible.
Similar Questions for you
Na+ C + N + S ®NaSCN
Fe3+ + SCN–
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AIF6 or CaF2 which lowers the melting point of the mixture and brings conductivity.
Ellingham diagram explains the feasibility of reduction process not the kinetics of process.
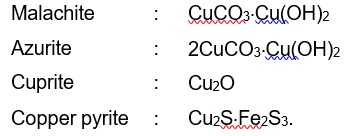
Malachite : CuCO3.Cu (OH)2
Azurite : 2CuCO3.Cu (OH)2
Cuprite : Cu2O
Copper pyrite : Cu2S.Fe2S3.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers