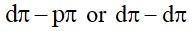6.4 Explain: (i) Zone refining (ii) Column chromatography.
6.4 Explain: (i) Zone refining (ii) Column chromatography.
-
1 Answer
-
Zone refining method is based on the principle that impurities are more soluble in the molten state of metal (the melt) than in the solid state. The impure metal is heated with the help of a circular mobile heater at one end. This results in the formation of the molten zone or melt. As the heater is removed along with the length of the rod, the pure metal crystallizes out of the melt and impurities pass into the adjacent molten zone. This process is repeated several times till the impurities are completely driven to the end of the rod which is then cut off and discarded. The method is very useful for semiconductor and other metals of v
...more
Similar Questions for you
Na+ C + N + S ®NaSCN
Fe3+ + SCN–
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AIF6 or CaF2 which lowers the melting point of the mixture and brings conductivity.
Ellingham diagram explains the feasibility of reduction process not the kinetics of process.
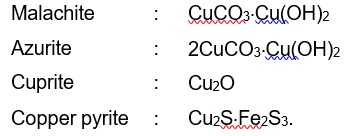
Malachite : CuCO3.Cu (OH)2
Azurite : 2CuCO3.Cu (OH)2
Cuprite : Cu2O
Copper pyrite : Cu2S.Fe2S3.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers