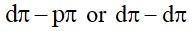6.4 Is it true that under certain conditions, Mg can reduce Al2O3 and Al can reduce MgO? What are those conditions?
6.4 Is it true that under certain conditions, Mg can reduce Al2O3 and Al can reduce MgO? What are those conditions?
-
1 Answer
-
Below 1683K, the melting point of silicon, the Δ fGo curve for the formation of SiO2 lies above the Δ fGo curve for MgO, so, at temperature below 1683 K, Mg can reduce SiO2. On the other hand, above 1683 K, the ΔfGo curve for MgO lies above ΔfGo curve for SiO2. Hence, at a temperature above 1683 K, Si can reduce MgO to Mg.

Similar Questions for you
Na+ C + N + S ®NaSCN
Fe3+ + SCN–
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AIF6 or CaF2 which lowers the melting point of the mixture and brings conductivity.
Ellingham diagram explains the feasibility of reduction process not the kinetics of process.
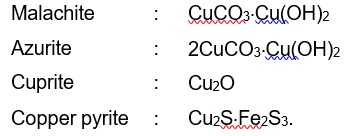
Malachite : CuCO3.Cu (OH)2
Azurite : 2CuCO3.Cu (OH)2
Cuprite : Cu2O
Copper pyrite : Cu2S.Fe2S3.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers