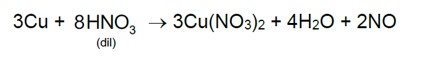7.31 Why is ICl more reactive than I2?
7.31 Why is ICl more reactive than I2?
-
1 Answer
-
7.31
In interhalogen compound ICl and I2 the atoms are bonded by covalent bonds
Reactivity refers to the rate at which a chemical species will undergo reaction in time. For reaction to take place the compound needs to be broken into separate elements first, then the individual elements react with other elements to form new compounds. So any compound which can easily break into their individual elements can react faster.
The covalent bonds between dissimilar atoms I and Cl atoms in ICl are weaker than between similar atoms in I2. Therefore the bond between ICL will break easily so the I and Cl atom will be easily available to form another
...more
Similar Questions for you
HClO4 is the most acidic compound.
Group number = 11 (Atomic number = 111)
Heavier element of p block do not from pπ− pπ bonds as their atomic orbital are so large and diffius that they cannot have effecitve overlapping.
The inertness of
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers