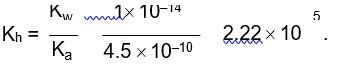7.50. The degree of ionization of a 0.1 M bromoacetic acid solution is 0.132. Calculate the pH of the solution and the PKa of bromoacetic acid.
7.50. The degree of ionization of a 0.1 M bromoacetic acid solution is 0.132. Calculate the pH of the solution and the PKa of bromoacetic acid.
H+] = cα = 0.1 * 0.132 = 0.0132M
pH = −log [H+] = −log0.0132
= 1.88
The acid dissociation constant is
Ka? = cα2? / (1−α) = 0.1 * (0.132)2 / (1−0.132)
= 2.01*10−3.
pKa? = −logKa? = −log (2.01*10−3) ≈ 2.7
Similar Questions for you
0.01 M NaOH,
M = 1 * 10-2
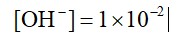
pOH = 2
pH = 2
Kp = Kc (RT)Dng
36 * 10–2 = Kc (0.0821 * 300)–1
Kc = 0.36 * 0.0821 * 300 = 8.86 » 9
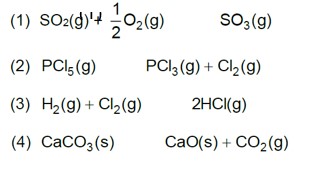
A(g) ->B(g) + (g)
Initial moles n 0 &nbs
On increasing pressure, equilibrium moves in that direction where number of gaseous moles decreases.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering