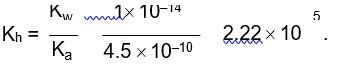7.58. The solubility of Sr(OH)2 at 298 K is 19.23 g/L of solution. Calculate the concentrations of strontium and hydroxyl ions and the pH of the solution.
7.58. The solubility of Sr(OH)2 at 298 K is 19.23 g/L of solution. Calculate the concentrations of strontium and hydroxyl ions and the pH of the solution.
Solubility of Sr (OH)2=19.23g/L
The molecular weight of Sr (OH)2 is 87.6 + 2 (17)=121.6
Then, concentration of Sr (OH)2=19.23 /121.63M=0.1581M
Sr (OH)2 (aq)→Sr2+ (aq)+2 (OH−) (aq)
∴ [Sr2+]=0.1581M
[OH−]=2*0.1581M=0.3126M
Now, Kw= [OH−] [H+]
=> [H+] = 10−14 / 0.3126
=> [H+]=3.2*10−14
∴pH= 13.495
Similar Questions for you
0.01 M NaOH,
M = 1 * 10-2
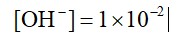
pOH = 2
pH = 2
Kp = Kc (RT)Dng
36 * 10–2 = Kc (0.0821 * 300)–1
Kc = 0.36 * 0.0821 * 300 = 8.86 » 9
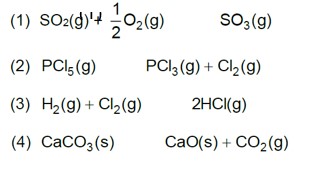
A(g) ->B(g) + (g)
Initial moles n 0 &nbs
On increasing pressure, equilibrium moves in that direction where number of gaseous moles decreases.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering