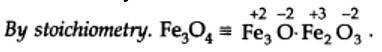8.2. What is the oxidation number of the underlined elements in each of the following and how do you rationalise your results?
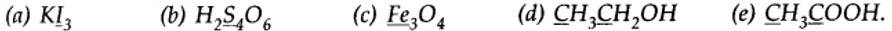
8.2. What is the oxidation number of the underlined elements in each of the following and how do you rationalise your results?
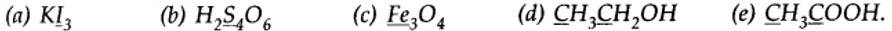
-
1 Answer
-
(a) In Kl3, since the oxidation number of K is +1, therefore, the average oxidation number of iodine = -1/3. But the oxidation number cannot be fractional. Therefore, we must consider its structure, K+ [I —I < I]–. Here, a coordinate bond is formed between I2 molecule and I– ion. The oxidation number of two iodine atoms forming the I2 molecule is zero, while that of iodine forming the coordinate bond is -1. Thus, the oxidation number of the three I atoms, atoms in Kl3 is 0, 0 and -1, respectively.
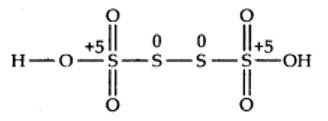
(b) By conventional method O.N. of S in H2S4O6is calculated as:
2 (+1) +4x + 6) (-2) = 0
Or x = +2.5
But all the four
...more
Similar Questions for you
Kindly go through the solution
(c) Li
Kindly go through the solution
(c) Al
Kindly go through the solution
(d) +6
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers