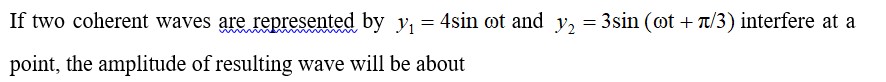8.38 What are inner transition elements? Decide which of the following atomic numbers are the atomic numbers of the inner transition elements : 29, 59, 74, 95, 102, 104.
8.38 What are inner transition elements? Decide which of the following atomic numbers are the atomic numbers of the inner transition elements : 29, 59, 74, 95, 102, 104.
8.38 The inner transition elements are those in which the last electron enters the f-subshell. The lanthanoids atomic number 58-71 and actinoids 90-103. The atomic numbers 59, 95 and 102 are inner transition elements
Similar Questions for you
K2Cr2O7 + H2O2 + H2SO4->
Potassium permanganate in alkaline medium oxidise lodide to lodate.
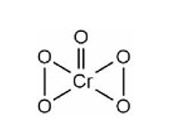
Compound A is
KMnO4 decomposes upon heating at 513 K and forms K2MnO4 and MnO2.
2KMnO4
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering