8.4. Fluorine reacts with ice and results in the change:
H2O(s) + F2 (g) ——> HF (g) + HOF (g)
Justify that this reaction is a redox reaction.
8.4. Fluorine reacts with ice and results in the change:
H2O(s) + F2 (g) ——> HF (g) + HOF (g)
Justify that this reaction is a redox reaction.
-
1 Answer
-
Writing the O.N. of each atom above its symbol, we have,
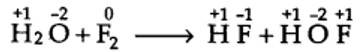
here, the O.N. of F decreases from 0 in F2 to -1 in HF and increases from 0 in F2 to +1 in HOF. Therefore, F2 is both reduced as well as oxidised. Thus, it is a redox reaction and more specifically, it is a disproportionation reaction.
Similar Questions for you
Kindly go through the solution
(c) Li
Kindly go through the solution
(c) Al
Kindly go through the solution
(d) +6
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
