8.9. Consider the reactions:
(a) 6CO2(g) + 6H2O(l) →C6H12O6(s) + 6O2(g)
(b) O3(g) + H2O2(l) →H2O(l) + 2O2(g)
Why it is more appropriate to write these reactions as:
(a) 6CO2(g) + 12H2O(l) →C6H12O6(s) + 6H2O(l) + 6O2(g)
(b) O3(g) + H2O2 (l) →H2O(l) + O2(g) + O2(g)
Also suggest a technique to investigate the path of above (a) and (b) redox reactions.
8.9. Consider the reactions:
(a) 6CO2(g) + 6H2O(l) →C6H12O6(s) + 6O2(g)
(b) O3(g) + H2O2(l) →H2O(l) + 2O2(g)
Why it is more appropriate to write these reactions as:
(a) 6CO2(g) + 12H2O(l) →C6H12O6(s) + 6H2O(l) + 6O2(g)
(b) O3(g) + H2O2 (l) →H2O(l) + O2(g) + O2(g)
Also suggest a technique to investigate the path of above (a) and (b) redox reactions.
-
1 Answer
-
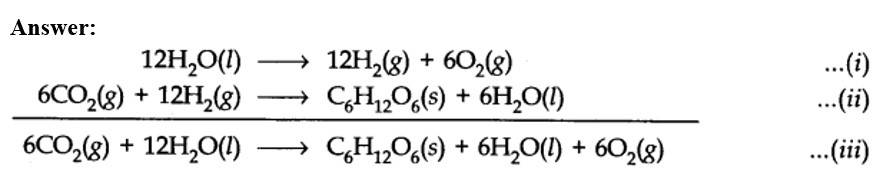
(a) Therefore, it is more appropriate to write the equation for photosynthesis as (iii) because it emphasises that 12H2O are used per molecule of carbohydrate formed and 6H2O are produced during the process.
(b) The purpose of writing O2 two times suggests that O2 is being obtained from each of the two reactants.
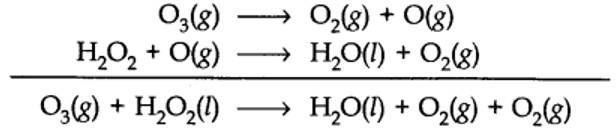 The path of reactions (a) and (b) can be determined by using H2O218 or D2O in reaction
The path of reactions (a) and (b) can be determined by using H2O218 or D2O in reaction
(a) or by using H2O218 or O318in reaction (b).
Similar Questions for you
Kindly go through the solution
(c) Li
Kindly go through the solution
(c) Al
Kindly go through the solution
(d) +6
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
