9.18 List various types of isomerism possible for coordination compounds, giving an example of each.
9.18 List various types of isomerism possible for coordination compounds, giving an example of each.
-
1 Answer
-
Isomers are the compounds which have same chemical formula but different arrangement of atoms in space. There are principle two types of isomerism:
(i) Stereo isomerism
(ii) Geometrical isomerism
(iii) Optical isomerism
(iv) Structural isomerism
(v) Ionisation isomerism
(vi) Linkage isomerism
(vii) Coordination isomerism
(viii) Solvate isomerism
Geometrical isomerism comes into existence by the different spatial arrangement of groups around the central metal atom. Similar groups may either be arranged on the same side or on opposite sides of the central metal atom. This gives rise to two types of isomers called cis and trans isomers. Wh
...more
Similar Questions for you
CoCl3.NH3 + AgNO3
x = 5
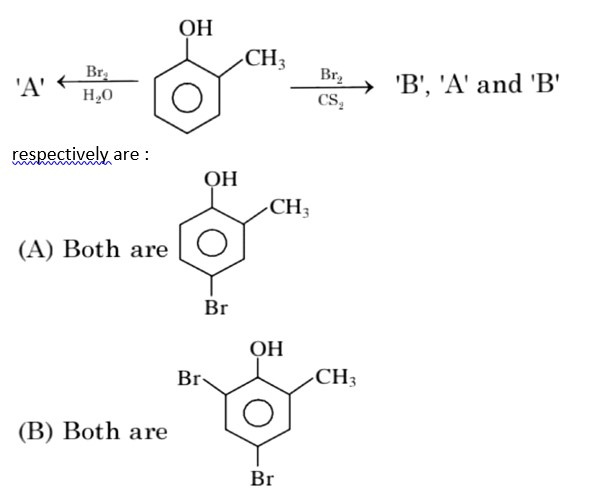
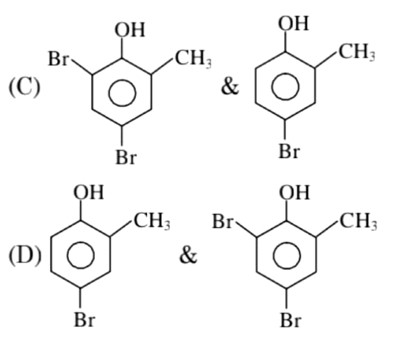
In H2O (polar solvent) dibromophenol derivative and in CS2 (non-polar solvent moneobromo phenol derivate is obtained.
3d => 4d => 5d CFSE increases for the same ligands.
Factual
⇒ leaching methods is used for those metal in which metal is more soluble than impurities and these are Al, Au, Ag, low grade Cu
σ bonded organometallic compound ⇒ M – C
σ-bond
and in π – bonded organo metallic compound
M – C
π bond
In ferrocene, there is π-bond
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers