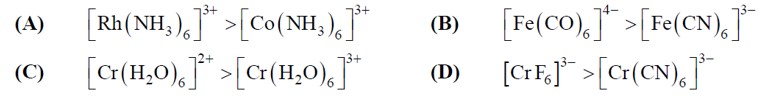9.20 Draw the structures of optical isomers of: (i) [Cr(C2O4)3] 3– (ii) [PtCl2(en)2] 2+ (iii) [Cr(NH3)2Cl2(en)]+
9.20 Draw the structures of optical isomers of: (i) [Cr(C2O4)3] 3– (ii) [PtCl2(en)2] 2+ (iii) [Cr(NH3)2Cl2(en)]+
-
1 Answer
-
Optical isomers are the one which rotate the plane polarized light by a certain angle when passed through them and are mirror images of each other, also called as stereoisomer.
(i) C2O4 2- is a bidentate ligand so it can attach to central atom at two sites, forming a chelate ring. In the complexes of this type, three symmetrical bidentate chelating ligands AA are coordinated to the central metal atom M. Such complexes do not possess any element of symmetry and are optically active. Moreover, these complexes can be resolved into optical
(ii) In this complex Cl is an unidentate ligand with one site of attachment whereas -en (ethyle
...more
Similar Questions for you
CoCl3.NH3 + AgNO3
x = 5
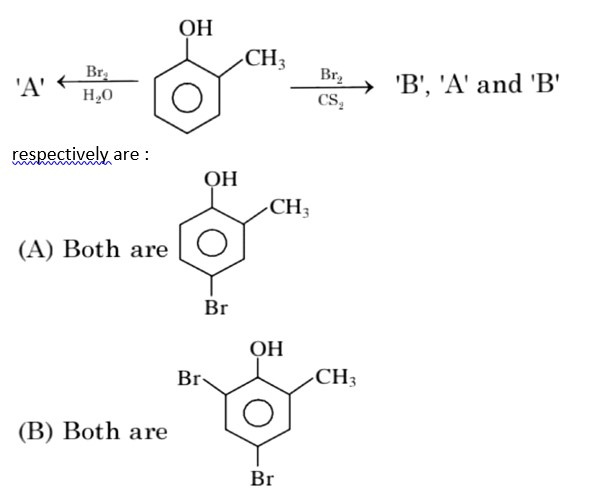
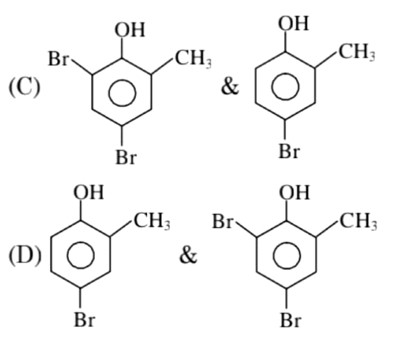
In H2O (polar solvent) dibromophenol derivative and in CS2 (non-polar solvent moneobromo phenol derivate is obtained.
3d => 4d => 5d CFSE increases for the same ligands.
Factual
⇒ leaching methods is used for those metal in which metal is more soluble than impurities and these are Al, Au, Ag, low grade Cu
σ bonded organometallic compound ⇒ M – C
σ-bond
and in π – bonded organo metallic compound
M – C
π bond
In ferrocene, there is π-bond
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers