9.32 Discuss the nature of bonding in metal carbonyls.
9.32 Discuss the nature of bonding in metal carbonyls.
-
1 Answer
-
Compounds containing carbonyl
Ligands only are known as homoleptic carbonyl. Such types of compounds are formed by most of the transition metals. These metal carbonyls always have simple, well-defined structures. In metal carbonyls the metal - carbonyl bond possess both s and p.character. M-C-bond is sigma bond. It is formed by the donation of lone pair of electrons of the carbonyl carbon into the vacant orbital of the metal. The M-C pi bond is formed by the donation of a pair of electron from a filled d orbital of a metal into the vacant antibonding? orbital of carbon monoxide. Such type of metal to ligand bonding creates a synergic ef
...more
Similar Questions for you
CoCl3.NH3 + AgNO3
x = 5
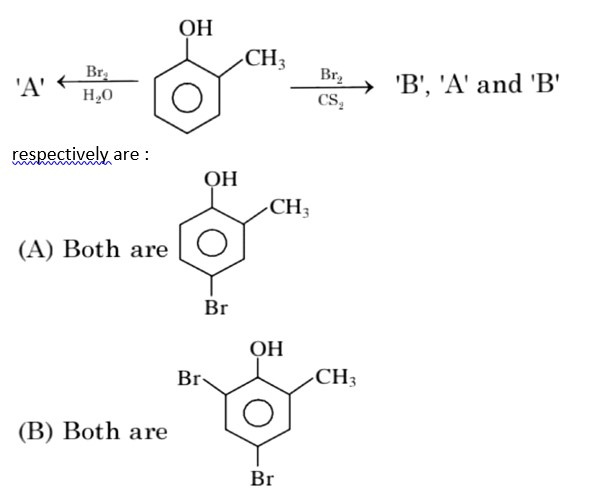
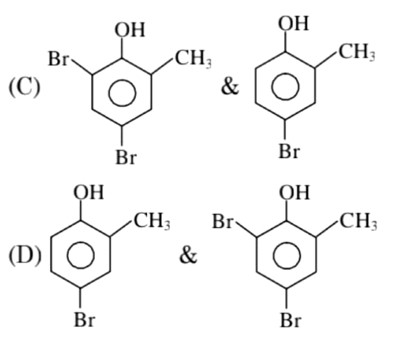
In H2O (polar solvent) dibromophenol derivative and in CS2 (non-polar solvent moneobromo phenol derivate is obtained.
3d => 4d => 5d CFSE increases for the same ligands.
Factual
⇒ leaching methods is used for those metal in which metal is more soluble than impurities and these are Al, Au, Ag, low grade Cu
σ bonded organometallic compound ⇒ M – C
σ-bond
and in π – bonded organo metallic compound
M – C
π bond
In ferrocene, there is π-bond
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers