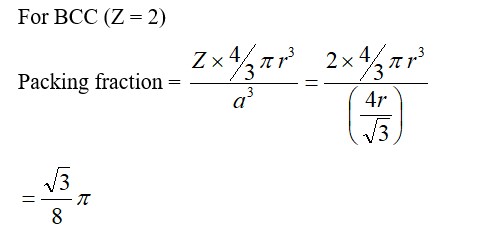A 1 molal K4[Fe(CN)6] solution has a degree of dissociation of 0.4. Its boiling point is equal to that of another solution which contains 18.1 weight percent of a non electrolytic solute A. The molar mass of A is ______ u. (Round off to the nearest integer).
[Density of water = 1.0 g cm?³]
A 1 molal K4[Fe(CN)6] solution has a degree of dissociation of 0.4. Its boiling point is equal to that of another solution which contains 18.1 weight percent of a non electrolytic solute A. The molar mass of A is ______ u. (Round off to the nearest integer).
[Density of water = 1.0 g cm?³]
For the dissociation of K? [Fe (CN)? ]? 4K? + [Fe (CN)? ]? , the number of ions produced (n) is 5.
The degree of dissociation (α) is related to the van't Hoff factor (i) by α = (i-1)/ (n-1).
Given α = 0.4: 0.4 = (i - 1) / (5 - 1) => 1.6 = I - 1 => I = 2.6
Using the depression in freezing point formula
Similar Questions for you
ΔG° = –RT * 2.303 log K
–nFE° = +RT * 2.303 log K
2 * 96500 * 0.295 = 8.314 * 298 * 2.303 log10 K
10 = log10 K = 1010
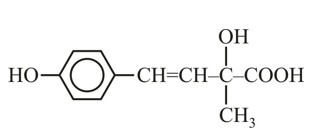
It has chiral centre and differently di substituted double bonded carbon atoms.
For FCC lattice
Packing efficiency = 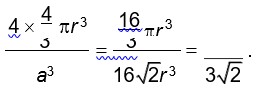
CsCl has BCC structure in which Cl– is present at corners of cube and Cs+ at body centre
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering