A box contains 0.90g of liquid water in equilibrium with water vapour at 27°C. The equilibrium vapour pressure of water at 27°C is 32.0 Torr. When the volume of the box is increased, some of the liquid water evaporates to maintain the equilibrium pressure. If all the liquid water evaporates, then the volume of the box must be________ litre. [Nearest Integer]
(Given : R = 0.082 L atm K-1 mole-1)
(Ignore the volume of the liquid water and assume water vapours behave as an ideal gas.)
A box contains 0.90g of liquid water in equilibrium with water vapour at 27°C. The equilibrium vapour pressure of water at 27°C is 32.0 Torr. When the volume of the box is increased, some of the liquid water evaporates to maintain the equilibrium pressure. If all the liquid water evaporates, then the volume of the box must be________ litre. [Nearest Integer]
(Given : R = 0.082 L atm K-1 mole-1)
(Ignore the volume of the liquid water and assume water vapours behave as an ideal gas.)
Similar Questions for you
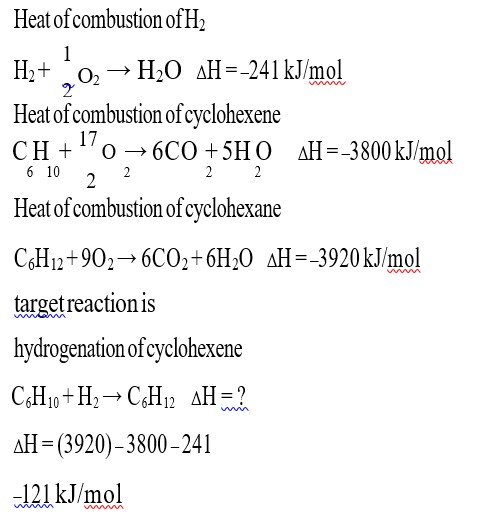
CH3COOH + NaOH → CH3COONa + H2O
ΔH = –50.6 kJ/mol
NaOH + SA [HCl] → NaCl + H2O
ΔH = –55.9 kJ/mol
the value of ΔH for ionisation of CH3COOH
⇒ ΔH = +55.9 – 50.6
5.3 kJ/mol
Kindly consider the solution
Fact.
Kindly go through the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering