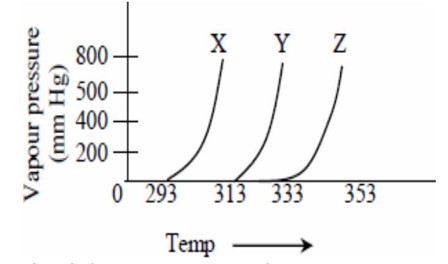
A graph of vapour pressure and temperature for three different liquids , and is shown below:
The following inferences are made :
has higher intermolecular interactions compared to
has lower intermolecular interactions compared to
has lower intermolecular interactions compared to
The correct inference (s) is / are :
A graph of vapour pressure and temperature for three different liquids , and is shown below:
The following inferences are made :
has higher intermolecular interactions compared to
has lower intermolecular interactions compared to
has lower intermolecular interactions compared to
The correct inference (s) is / are :
Option 1 -
(c)
Option 2 -
(a) and (c)
Option 3 -
(b)
Option 4 -
(a)
-
1 Answer
-
Correct Option - 3
Detailed Solution:At a fixed temperature, having more vapour pressure as compared to . So, intermolecular interaction is lower as compared to .
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers