A sample of hydrogen atoms de-excites from 6th excited state to the ground state in one or more electronic transitions. If no spectral line is obtained in the Paschen and Brackett series, then the maximum number of spectral lines of different photon energies obtained will be-
A sample of hydrogen atoms de-excites from 6th excited state to the ground state in one or more electronic transitions. If no spectral line is obtained in the Paschen and Brackett series, then the maximum number of spectral lines of different photon energies obtained will be-
Option 1 - <p>21</p>
Option 2 - <p>15</p>
Option 3 - <p>10</p>
Option 4 - <p>7</p>
3 Views|Posted 5 months ago
Asked by Shiksha User
No answers yet.
Can you answer this question?Similar Questions for you
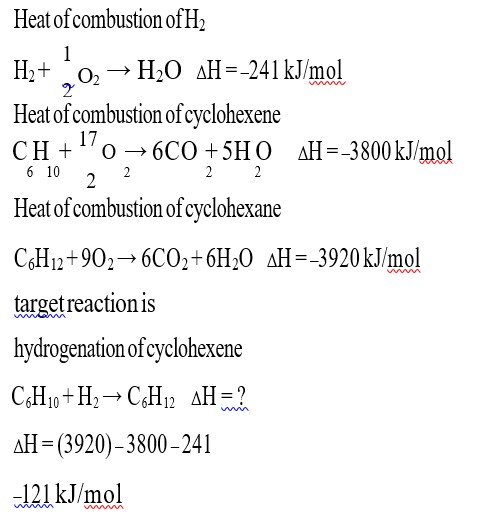
CH3COOH + NaOH → CH3COONa + H2O
ΔH = –50.6 kJ/mol
NaOH + SA [HCl] → NaCl + H2O
ΔH = –55.9 kJ/mol
the value of ΔH for ionisation of CH3COOH
⇒ ΔH = +55.9 – 50.6
5.3 kJ/mol
Kindly consider the solution
Fact.
Kindly go through the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering