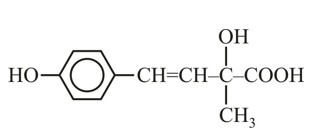
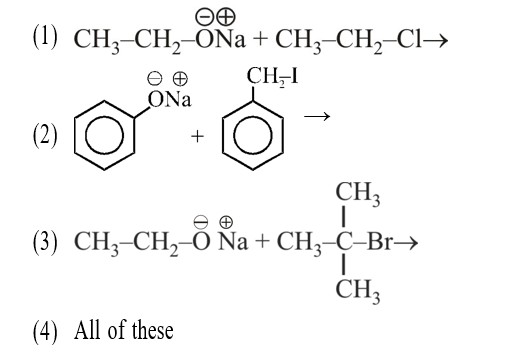
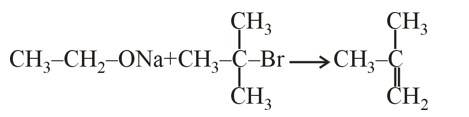
A solution contains 1 g mol each of p-toluene diazonium chloride and p-nitrophenyl diazonium chloride. To this 1 g mol of alkaline solution of phenol is added. Predict the major product. Explain your answer.
A solution contains 1 g mol each of p-toluene diazonium chloride and p-nitrophenyl diazonium chloride. To this 1 g mol of alkaline solution of phenol is added. Predict the major product. Explain your answer.
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
This is an example of an electrophilic aromatic substitution reaction. phenol generates phenoxide ion in alkaline medium, which is more electron-rich than phenol and thus more reactive to electrophilic attack. In this reaction, the el
Similar Questions for you
ΔG° = –RT * 2.303 log K
–nFE° = +RT * 2.303 log K
2 * 96500 * 0.295 = 8.314 * 298 * 2.303 log10 K
10 = log10 K = 1010
It has chiral centre and differently di substituted double bonded carbon atoms.
Rate of ESR ∝ No. of α – H (Hyperconjugation)
Cr3+ion is a most stable in aqueous solution due to. t2g half filled configuration
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering