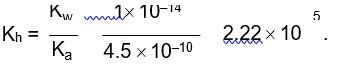A sparingly soluble salt having general formula Ap+x Bq- y and molar solubility S is in equilibrium with its saturated solution. Derive a relationship between the solubility and solubility product for such salt.
A sparingly soluble salt having general formula Ap+x Bq- y and molar solubility S is in equilibrium with its saturated solution. Derive a relationship between the solubility and solubility product for such salt.
The equation can be written as-
Ap+x + Bq- y ? xAp+ (aq) + yBq-
S moles of A and B dissolves to give x S moles of Ap+ and y S moles of Bq-.
Ksp = [Ap+]x [Bq-]y = [x5]x [y5]y
= xx y y 5 x+y
Similar Questions for you
0.01 M NaOH,
M = 1 * 10-2
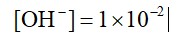
pOH = 2
pH = 2
Kp = Kc (RT)Dng
36 * 10–2 = Kc (0.0821 * 300)–1
Kc = 0.36 * 0.0821 * 300 = 8.86 » 9
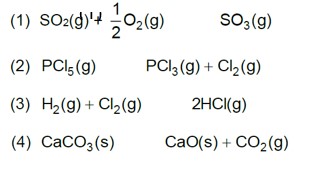
A(g) ->B(g) + (g)
Initial moles n 0 &nbs
On increasing pressure, equilibrium moves in that direction where number of gaseous moles decreases.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering