According to Arrhenius equation rate constant k is equal to Ae - . Which of the following options represents the graph of in k vs .
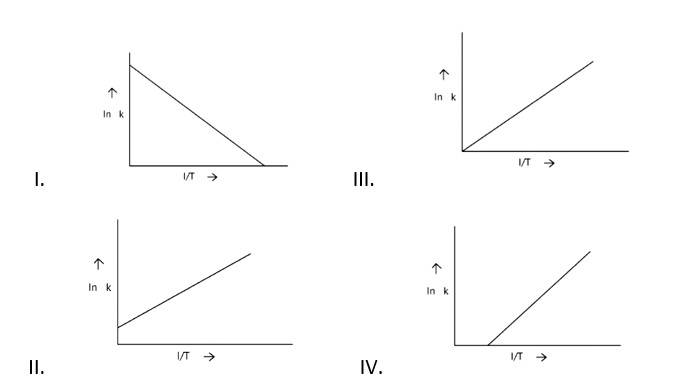
According to Arrhenius equation rate constant k is equal to Ae - . Which of the following options represents the graph of in k vs .

-
1 Answer
-
This is a Fill in the blanks Type Question as classified in NCERT Exemplar
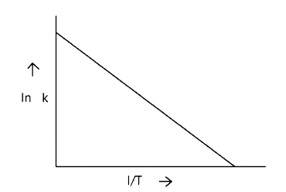
Ans: Correct option A
Arrhenius equation Ae -
K = rate constant
A= frequency factor
Ea= Activation Energy
R= gas constant
T= temperature
ln k = lnA -
When the temperature rises, ln falls.
∴ ln kv/s , is a negative slope.
ln A is intercepted by k, and its magnitude decreases with time.
∴ Negative slope is obtained.
When compared to the other possibilities, T is increasing over time, which is incorrect.
ln k = + lnA
Similar Questions for you
Kindly go through the solution
Ea = 216.164kJ/mol 216
Reaction rate is used to measure how fast or slow reactions occur per unit time. The rate constant is a proportionality factor that remains constant for every reaction.
Yes, in elementary reactions, order and molecularity can be the same, but this is not always the case because order is an experimental quantity, and molecularity is a theoretical concept.
Reaction Kinetics, also known as chemical kinetics, is the study of the rate of chemical reaction and the factors affecting the reaction rate, such as temperature, concentration, and catalyst.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
