According to the valence bond theory the hybridization of central metal atom is dsp2 for which one of the following compounds?
According to the valence bond theory the hybridization of central metal atom is dsp2 for which one of the following compounds?
Option 1 -
K2[Ni(CN)4]
Option 2 -
NiCl2.6H2O
Option 3 -
Na2[NiCl4]
Option 4 -
[Ni(CO)4]
-
1 Answer
-
Correct Option - 1
Detailed Solution: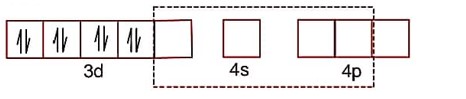
dsp2 hybridisation
Paring developed due to strong ligand effect.
NiCl2.6H2O
Ni2+ ->3d84s0
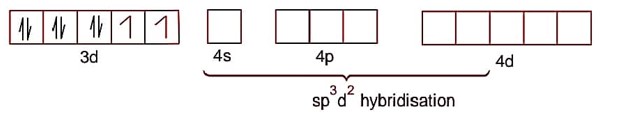
Na 2[NiCl 4] Ni2+ -> 3d84s0
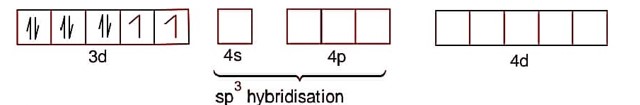
[Ni (CO)4]
Ni->3d84s2
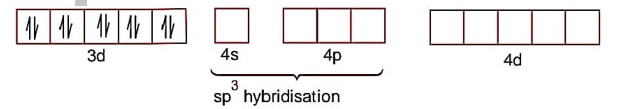 Paring developed due to strong ligand (CO).
Paring developed due to strong ligand (CO).
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
