Amongst the following elements whose electronic configurations are given below, the one having the highest ionisation enthalpy is
(i) [Ne]3s2 3p1
(ii) [Ne]3s2 3p3
(iii) [Ne]3s2 3p2
(iv) [Ar]3d104s24p3
Amongst the following elements whose electronic configurations are given below, the one having the highest ionisation enthalpy is
(i) [Ne]3s2 3p1
(ii) [Ne]3s2 3p3
(iii) [Ne]3s2 3p2
(iv) [Ar]3d104s24p3
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (ii)
Aluminium has [Ne]3s2 3p1 electronic configuration. Phosphorus has [Ne]3s2 3p3. The electronic configuration of silicon is [Ne]3s2 3p2 and the electronic configuration of arsenic is [Ar]3d104s24p3.
Ionisation ene
Similar Questions for you
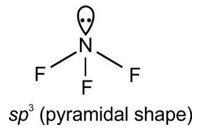
He2 has zero bond order hence it does not exist.
The three fundamental laws of chemistry are - Law of Definite Proportions, Law of Conservation of Mass, and Law of Multiple Proportions.
The three types of chemical bonds are - ionic, metallic and covalent bonds. When the electrons transfer between the atoms, they form the Ionic bonds by producing charged ions that are attracted to each other. When atoms share electrons, covalent bonds are created. When metal atoms share a sea of del
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Four 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering