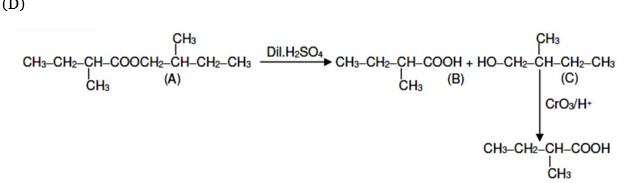
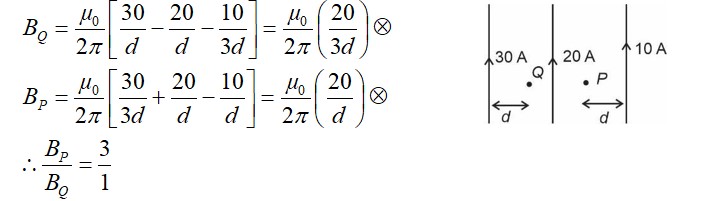An organic compound [A], molecular formula C??H??O?, was hydrolyzed with dilute sulphuric acid to give a carboxylic acid [B] and an alcohol [C]. Oxidation of [C] with CrO? – H?SO? produced [B]. Which of the following structures are not possible for [A]?
An organic compound [A], molecular formula C??H??O?, was hydrolyzed with dilute sulphuric acid to give a carboxylic acid [B] and an alcohol [C]. Oxidation of [C] with CrO? – H?SO? produced [B]. Which of the following structures are not possible for [A]?
Option 1 -
CH₃)₃C-COOCH₂C(CH₃)₃
Option 2 -
CH₃CH₂CH₂COOCH₂CH₂CH₂CH₃
Option 3 -
CH₃-CH₂-CH(CH₃)-COOCH₂-CH(CH₃)-CH₂CH₃
Option 4 -
CH₃-CH₂-CH(CH₃)-OCOCH₂CH(CH₃)-CH₂CH3
-
1 Answer
-
Correct Option - 4
Detailed Solution:(B) contains eight carbon atoms whereas the molecular formula C? H? O? contains ten carbon atoms.
Similar Questions for you
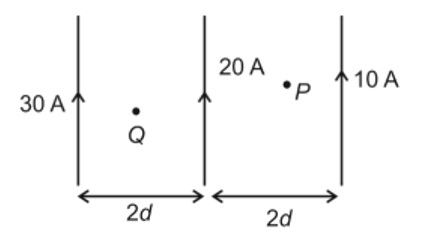
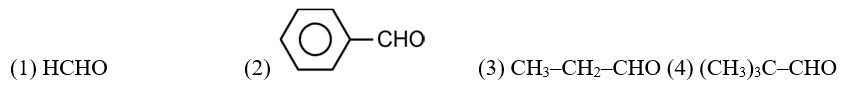
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers