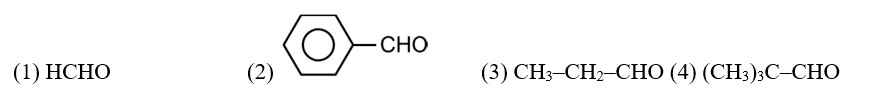
An organic compound with the molecular formula C9H10O forms 2,4-DNP derivative, reduces Tollens’ reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. Identify the compound.
An organic compound with the molecular formula C9H10O forms 2,4-DNP derivative, reduces Tollens’ reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. Identify the compound.
-
1 Answer
-
Since the given compound with molecular formula C9H10O form a 2,4-DNP derivative and reduce Tollen's reagent, it must be an aldehyde. Since it undergoes cannizzaro reaction, therefore CHO group is directly attached to the benzene ring.
Since on vigorous oxidation, it gives 1,2-benzene dicarboxylic acid, therefore it must be ortho-substituted benzaldehyde. The only o-substituted aromatic aldehyde having molecular formula C9H10O is o—ethyl benzyldehyde all the reaction can show now be explained on the basis of this structure-
Similar Questions for you
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers