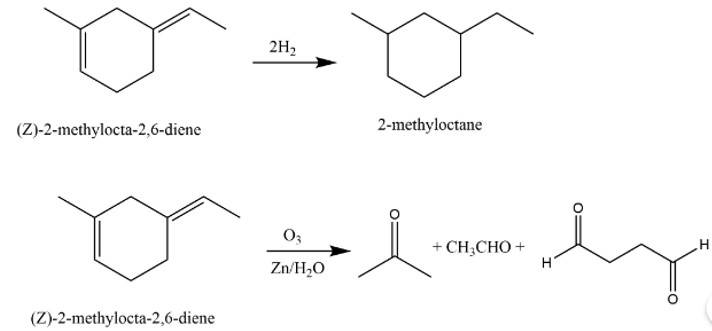
An unsaturated hydrocarbon ‘A’ adds two molecules of H2 and on reductive ozonolysis gives butane-1,4-dial, ethanal and propanone. Give the structure of ‘A’, write its IUPAC name and explain the reactions involved.
An unsaturated hydrocarbon ‘A’ adds two molecules of H2 and on reductive ozonolysis gives butane-1,4-dial, ethanal and propanone. Give the structure of ‘A’, write its IUPAC name and explain the reactions involved.
-
1 Answer
-
This is a long answer type question as classified in NCERT Exemplar
The structure of A is
The reaction involved are as follows:
Similar Questions for you
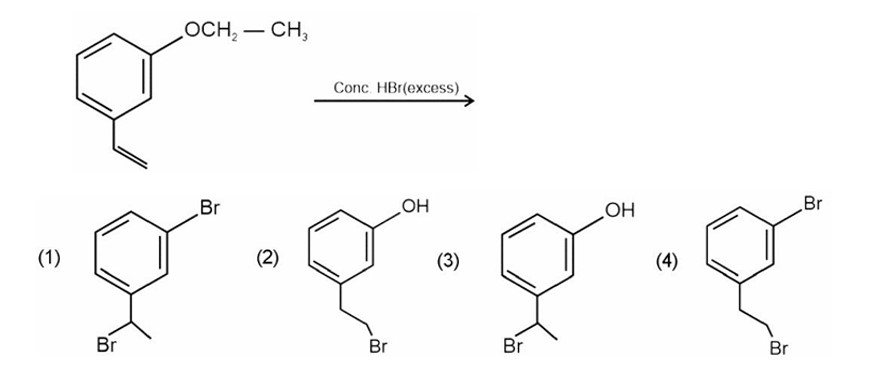
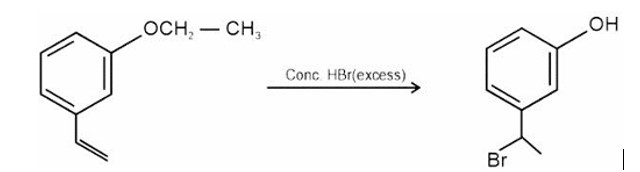
HBr adds to alkene in accordance with Markovnikov's rule.
Delocalisation of
To study Hydrocarbons for NEET, you can use the Hydrocarbons Class 11th NCERT solutions PDF.
Alkanes, Alkenes, Alkynes, and Aromatics hydrocarbons are the four main hydrocarbons.
Hydrocarbons are organic compounds made of only carbon and hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers