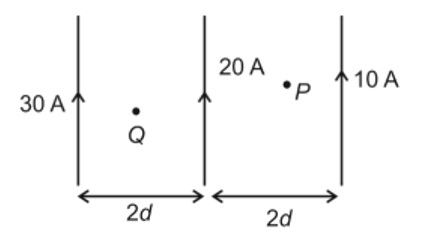
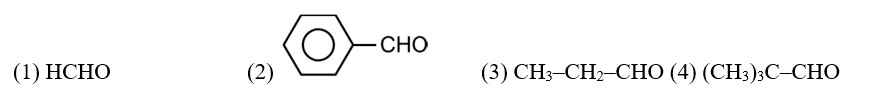
Arrange the following compounds in increasing order of their boiling points. CH3 CHO, CH3 CH2OH, CH3OCH3 , CH3 CH2 CH3
Arrange the following compounds in increasing order of their boiling points. CH3 CHO, CH3 CH2OH, CH3OCH3 , CH3 CH2 CH3
-
1 Answer
-
The molecular masses of the given compounds are in the range of 44-66 g/mol. The second molecule, CH3CH2OH contains OH alcoholic group due to which it undergoes extensive H bonding with each other molecules, leading to the association of the molecules. Therefore, it has the highest boiling point. In the molecule CH3CHO, there is strong intermolecular dipole-dipole attraction due to presence of - CHO aldehydic group ( as H will have partial positive charge and oxygen will have partial negative charge, so there will attraction between molecules), which is weak in case of CH3OCH3 as both CH3 groups have +I effect which results in decrease
...more
Similar Questions for you
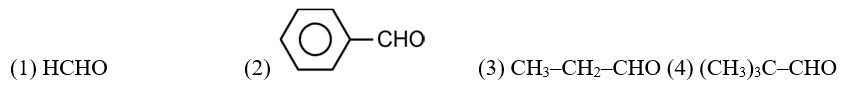
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers