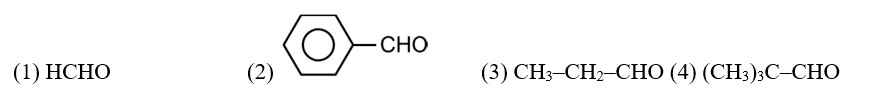
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
(i) Ethanal, Propanal, Propanone, Butanone.
(ii) Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
(i) Ethanal, Propanal, Propanone, Butanone.
(ii) Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
-
1 Answer
-
(i) Ethanal, Propanal, Propanone, Butanone
The +I effect increases in the order as:
Ethanal
Therefore, the electron density of the carbonyl carbon increases as the +I effect due to the alkyl group increases. As a result, the chances of attack by a nucleophile (which are rich in electrons and carry a negative charge, not necessarily usually have a lone pair of an electrons) decrease because the carbonyl carbon has electron density on it. Hence, the increasing order of the reactivity of the given compounds in nucleophilic addition reaction is:
Ethanal > Propanal> Propanone > Butanone.
(ii) Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde,
...more
Similar Questions for you
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers